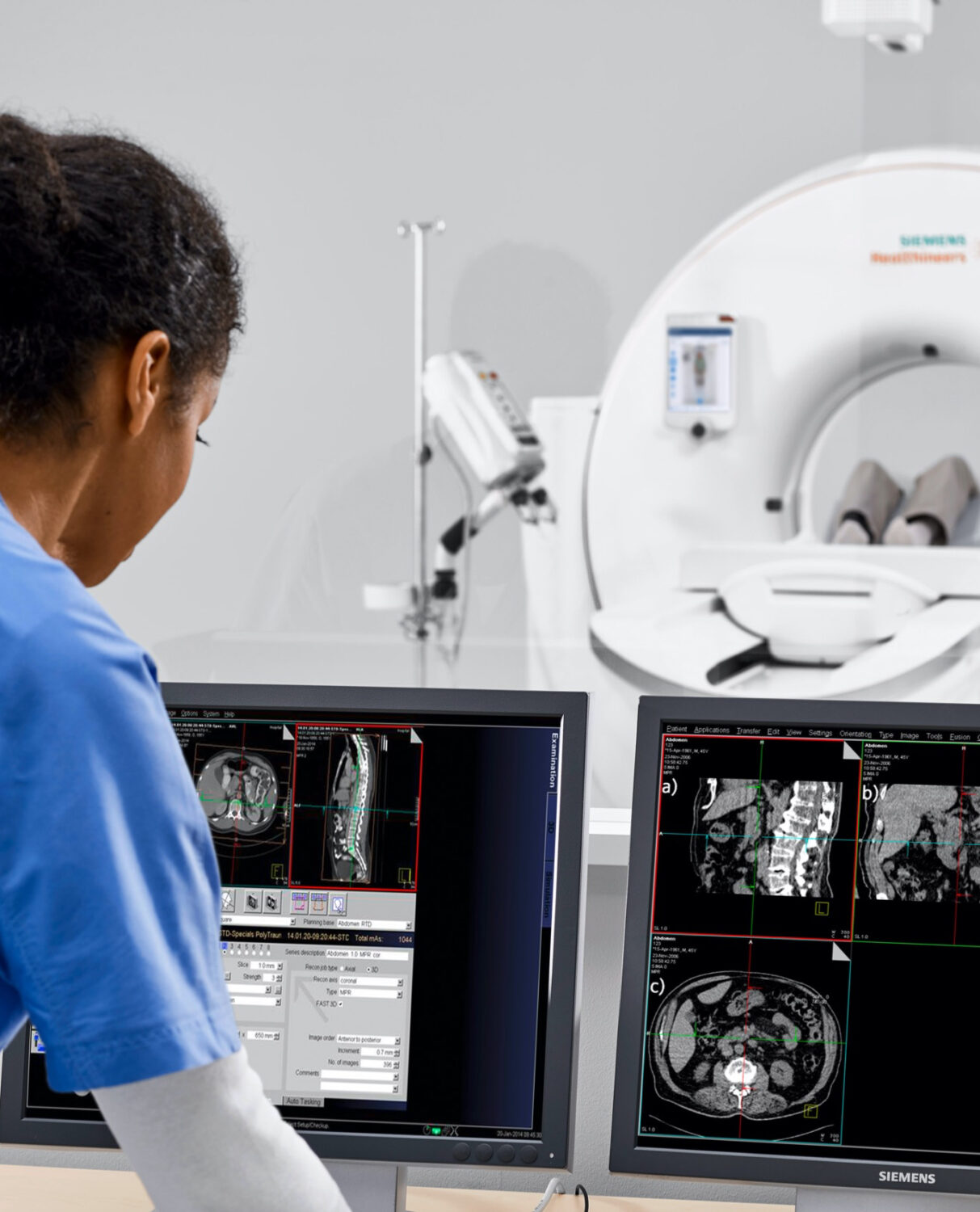
Ariane Jäger and Cornelia Lüderitz clarify what is meant by the two terms and what added value can be generated through the consistent use of UX methods. We give an overview of the user-centred design process, which takes into account the perspectives of many stakeholders involved in the development of medical devices: Users (patients / professionals), developers, designers, risk managers, project managers and product owners. We also present a practical guide using the example of the radiology software AI-RAD Companion from Siemens Healthineers, which provides answers to questions in the planning of certification processes: How do I elicit user needs and possible risks on the product? How do I translate these into concrete product requirements? What do I have to consider in the documentation?
The event will take place under the umbrella of the Arbeitskreis Software-Qualität und -Fortbildung e.V. (ASQF). As a network, the ASQF connects high-performance start-ups, medium-sized companies, global players, universities and research institutions and develops proposals that take into account the new requirements of digitalisation.
We look forward to your questions at the event on Thursday, 6th May 2021, from 5:30 to 7 pm!
Registrations are possible here.
Image source: Copyright Siemens Healthineers